India’s patent authority has delivered a decisive blow in a high-stakes medical technology dispute. The Office of the Controller General of Patents, Designs and Trade Marks (CGPDTM) has revoked Indian Patent No. 323440, a patent previously granted to Adiuvo Diagnostics Private Ltd..
The revocation follows a post-grant opposition filed by MolecuLight Corp., a global developer of fluorescence imaging devices. The ruling invalidates all claims of the patent, citing lack of novelty and prior art disclosures.
The decision reshapes the competitive landscape in pathogen detection and fluorescence imaging technologies in India. It also reinforces the power of India’s post-grant opposition system as a corrective legal tool.
The Patent at the Center of the Dispute
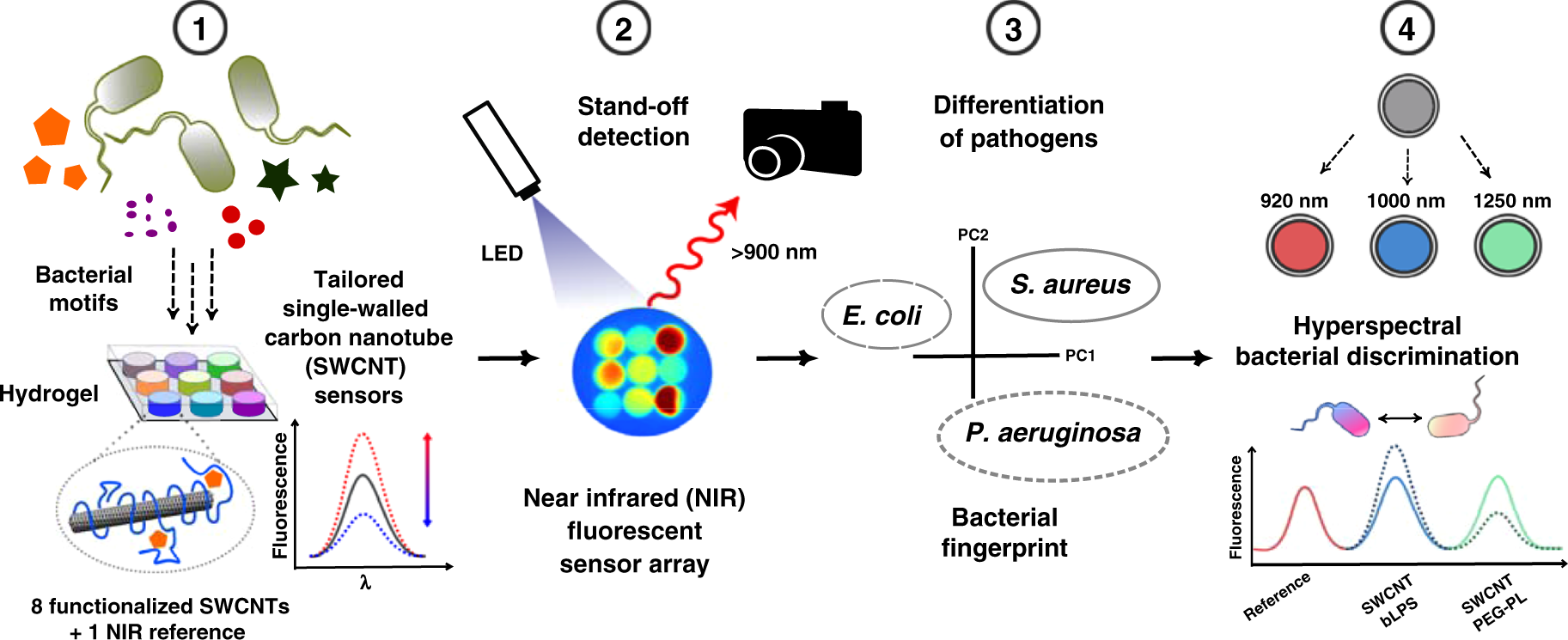
Indian Patent No. 323440 covered a “device and system for detection of time-dependent multi-spectral fluorescence response of pathogens.” The invention aimed to detect microbial presence by analyzing fluorescence signals emitted by pathogens under specific light conditions.
In simple terms, the technology focused on using light-based diagnostics to identify harmful microorganisms. Such systems promise faster detection. They also reduce reliance on traditional culture-based laboratory methods.
Adiuvo positioned the patent as a core intellectual property asset. The company sought to secure its standing in India’s growing medical diagnostics market. The patent formed part of a broader strategy to protect proprietary fluorescence-based detection methods.
However, MolecuLight challenged the patent soon after its grant.
MolecuLight’s Opposition Strategy
MolecuLight filed a post-grant opposition before the CGPDTM. The company argued that the patent lacked novelty and inventive step. It also cited earlier disclosures that allegedly anticipated Adiuvo’s claims.
The opposition relied heavily on prior art references, including patent applications that predated Adiuvo’s filing. MolecuLight contended that the claimed technology was not new. It argued that similar fluorescence-based pathogen detection systems already existed in publicly available documents.
The challenge was comprehensive. It attacked the patent’s core claims. It questioned the scientific basis. It also scrutinized the scope of protection sought.
India’s patent law permits such oppositions within a defined period after grant. This mechanism allows competitors, researchers, and industry players to challenge questionable patents.
In this case, the challenge succeeded.
CGPDTM’s Findings: Lack of Novelty and Inventive Step
After reviewing submissions from both sides, the CGPDTM concluded that all claims of Patent No. 323440 were unpatentable.
The authority found that the claims were anticipated by prior art. In patent law, anticipation means that earlier disclosures already describe the invention. If prior art exists, the invention cannot qualify as novel.
The Controller also determined that the claims lacked an inventive step. This requirement demands that an invention must not be obvious to a person skilled in the relevant field. The authority held that the technology did not clear this threshold.
As a result, the patent was revoked in its entirety.
This was not a partial amendment. It was a complete cancellation.
Comparative Analysis: Competing Positions
Adiuvo’s Position
Adiuvo defended its patent as an innovative diagnostic breakthrough. The company argued that its system offered a unique configuration for detecting time-dependent multi-spectral fluorescence responses.
It maintained that its technology improved detection accuracy. It emphasized efficiency gains. It framed the patent as a legitimate advancement over existing solutions.
MolecuLight’s Position
MolecuLight took a sharply different view. The company argued that the patent covered concepts already disclosed in earlier filings. It claimed that Adiuvo attempted to monopolize established fluorescence imaging principles.
MolecuLight’s strategy was direct. It focused on documentary evidence. It cited prior patents and applications. It demonstrated overlap in claimed features.
The CGPDTM ultimately sided with the opponent.
Market Impact: Who Gains and Who Loses?
The revocation shifts the balance of power.
For Adiuvo Diagnostics
The loss is significant. Patent protection offers exclusivity. It enables companies to block competitors. It strengthens licensing leverage.
Without Patent No. 323440, Adiuvo loses exclusive rights in India over the claimed fluorescence detection system. Competitors can now operate without fear of infringement under that specific patent.
The company may still hold other intellectual property assets. However, this ruling weakens its position in the fluorescence-based pathogen detection segment.
For MolecuLight
The decision represents a strategic win. It removes a potential barrier to market operations in India. It also reinforces the strength of MolecuLight’s own prior filings.
The ruling strengthens its freedom to operate. It protects its commercial interests. It sends a signal that its technology foundation predates the contested claims.
In competitive industries, such victories matter.
Broader Significance for India’s Patent Ecosystem
This case highlights the robustness of India’s post-grant opposition framework.
India allows pre-grant and post-grant oppositions. This dual system provides a powerful check against weak patents. It encourages scrutiny. It invites industry participation.
Critics sometimes argue that oppositions slow innovation. Supporters counter that they improve patent quality. They prevent monopolies based on incremental or obvious developments.
The revocation of Patent No. 323440 demonstrates that the system works. The authority conducted a detailed review. It examined evidence. It applied statutory tests. It delivered a reasoned decision.
For global medical device companies, the message is clear. Filing in India requires rigorous prior art analysis. Broad claims face close examination.
The Technology Angle: Fluorescence Imaging in Diagnostics
Fluorescence-based pathogen detection remains a rapidly evolving field. These systems illuminate tissues or samples with specific wavelengths of light. Pathogens emit fluorescence signals. Devices capture and analyze these responses.
The promise is speed and precision. Early detection reduces treatment delays. It improves clinical outcomes.
However, the field is crowded. Multiple companies invest in similar optical and imaging technologies. Patent disputes are common. Overlapping claims often trigger legal battles.
In such an environment, novelty becomes critical. Incremental improvements may not suffice.
Legal Lessons for Innovators
The revocation offers clear lessons:
- Conduct thorough prior art searches.
Overlooking existing disclosures can prove fatal. - Draft precise claims.
Broad claims invite challenge. Narrow, well-supported claims stand stronger. - Prepare for opposition.
In competitive sectors, opposition is not hypothetical. It is likely. - Document technical differentiation.
Inventive step must be demonstrable, not assumed.
Patent protection remains a powerful tool. But it demands precision and originality.
What Comes Next?
Adiuvo may explore legal remedies, including appeal options available under Indian law. Whether it chooses to do so remains unclear.
Meanwhile, the decision stands as a precedent. It reinforces the principle that patents must meet strict statutory standards. It also signals that India’s patent office will not hesitate to revoke claims that fail to qualify.
For the diagnostics industry, the ruling injects clarity. It removes uncertainty around Patent No. 323440. It opens competitive space. It resets the field.
Conclusion
The revocation of Indian Patent No. 323440 marks a decisive moment in India’s medical diagnostics patent landscape. The CGPDTM examined the evidence. It weighed competing arguments. It concluded that the patent lacked novelty and inventive step.
Adiuvo loses exclusivity. MolecuLight secures a strategic advantage. The market adjusts.
Most importantly, the decision underscores a fundamental truth. Patent rights are not permanent guarantees. They are conditional privileges. They survive only when innovation meets the highest legal standards.