Enveric Biosciences bolsters its position in the booming psychedelic-inspired therapeutics market. The company announces a major intellectual property win. The United States Patent and Trademark Office issues U.S. Patent No. 12,492,179.
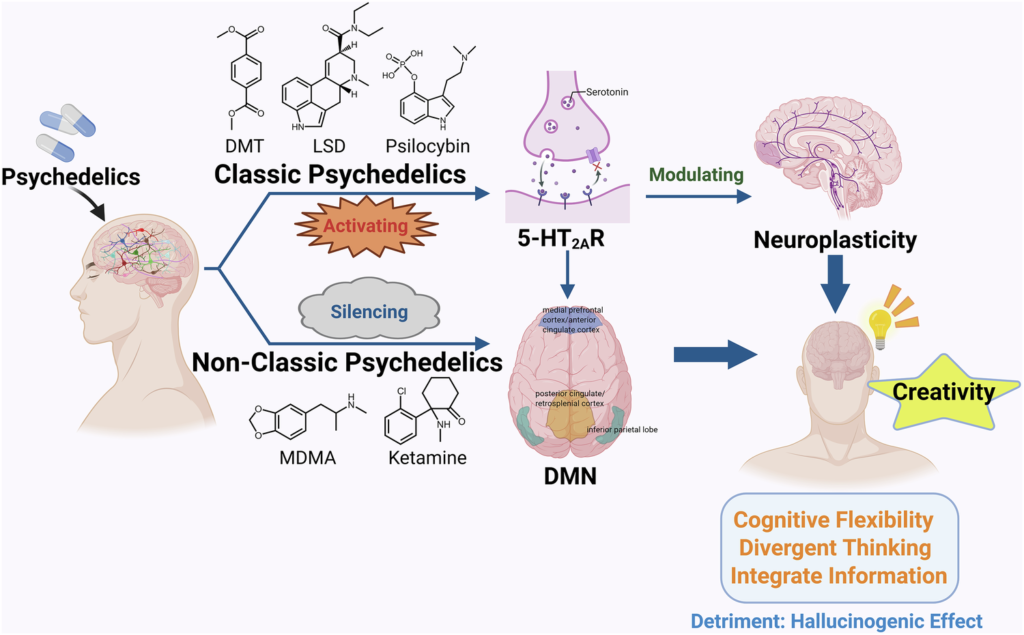
This patent covers novel molecules titled “Substituted Ethylamine Fused Heterocyclic Mescaline Derivatives.” Enveric designs these compounds to promote neuroplasticity. They target severe mental health disorders without causing hallucinations.
Patients struggle with depression, anxiety, PTSD, and addiction. Traditional treatments often fail. Enveric’s innovation addresses this gap. The new molecules derive from mescaline-like structures. Scientists modify them chemically. They enhance efficacy. They reduce side effects.
Hallucinogenic psychedelics show promise. Yet they pose challenges. Patients experience intense trips. Clinics require supervision. Regulators demand more data. Enveric avoids these hurdles. Its neuroplastogens deliver brain rewiring benefits. They skip the psychedelic experience.
Joseph Tucker, Ph.D., leads Enveric as CEO. He celebrates the milestone. “This patent expands our portfolio. It strengthens our pipeline. We target disorders with limited options. Our molecules interact with key receptors in novel ways. They promise better safety and outcomes.”
Composition-of-matter patents offer strong protection. They shield the core chemical structures. Enveric attracts partners. Big pharma seeks licensing deals. The company builds a competitive moat.
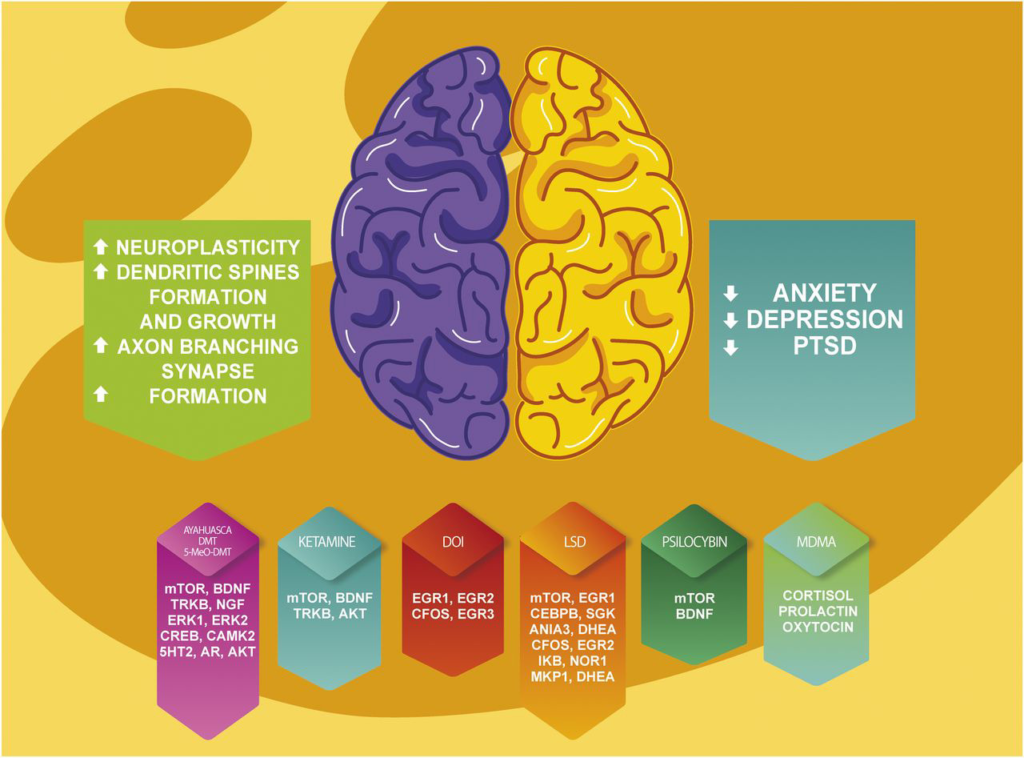
Enveric focuses on next-generation therapeutics. It develops small-molecule drugs. These promote neuroplasticity – the brain’s ability to form new connections. Neuroplasticity drives recovery in mental illness.
The psychedelic drugs market explodes. Analysts project growth from about $3-4 billion in 2024 to $8-10 billion by 2032. Compound annual rates hit 13-15%. Mental health crises fuel demand. Over 264 million people suffer depression worldwide. Treatment-resistant cases rise.
Companies chase non-hallucinogenic options. Enveric pioneers this shift. Its Psybrary™ platform generates thousands of candidates. Artificial intelligence aids discovery. The company holds dozens of patents. It issues more in 2025.
Enveric’s lead candidate shines. EB-003 advances rapidly. This first-in-class compound engages 5-HT2A and 5-HT1B receptors. It delivers fast antidepressant and anxiolytic effects. Preclinical data impress. Oral bioavailability works well. Brain penetration proves strong.
Enveric targets IND filing soon. Phase 1 trials follow. EB-003 treats outpatient settings. No need for therapists during sessions. Patients take pills at home. Convenience boosts adherence.
Other pipelines progress. EVM301 and EVM401 series expand. Notices of allowance arrive. Patents issue. Enveric licenses non-core assets. It funds core development.
Investors react positively. Shares jump in premarket trading after the December 29 announcement. The stock faces volatility. It trades as a micro-cap. Market cap hovers low. Yet milestones drive gains.
Enveric raises funds. Warrant exercises bring millions. Cash supports trials. The company regains Nasdaq compliance.
Experts praise the approach. Non-hallucinogenic drugs scale easier. They fit existing healthcare systems. Insurers cover standard pills. Clinics avoid psychedelic infrastructure.
Regulators warm to safer profiles. FDA grants breakthrough designations elsewhere. Enveric positions EB-003 for similar status.
Mental health innovation lags. Antidepressants date back decades. Side effects deter patients. Relapse rates stay high. Psychedelic research revives hope. Enveric refines it.
Researchers link neuroplasticity to recovery. Psychedelics boost dendritic growth. They increase synapses. Non-hallucinogenic versions isolate this mechanism.
Enveric collaborates. It presents at conferences. Data publications follow. Peer-reviewed papers validate methods.
Challenges remain. Clinical trials cost dearly. Enveric seeks partners. Out-licensing generates revenue.
The field attracts talent. Investors eye neuroplastogens. Enveric leads with patents and data.
Patients await better options. Suicide rates climb. Addiction devastates families. Enveric aims to help.
This patent marks progress. Enveric executes strategy. It builds value. The future looks brighter for mental health treatment.
Enveric Biosciences trades on Nasdaq as ENVB. It operates from Cambridge. The team drives innovation. They transform lives.
The mental health revolution gains speed. Enveric rides the wave. Non-hallucinogenic therapies emerge. Hope grows.





